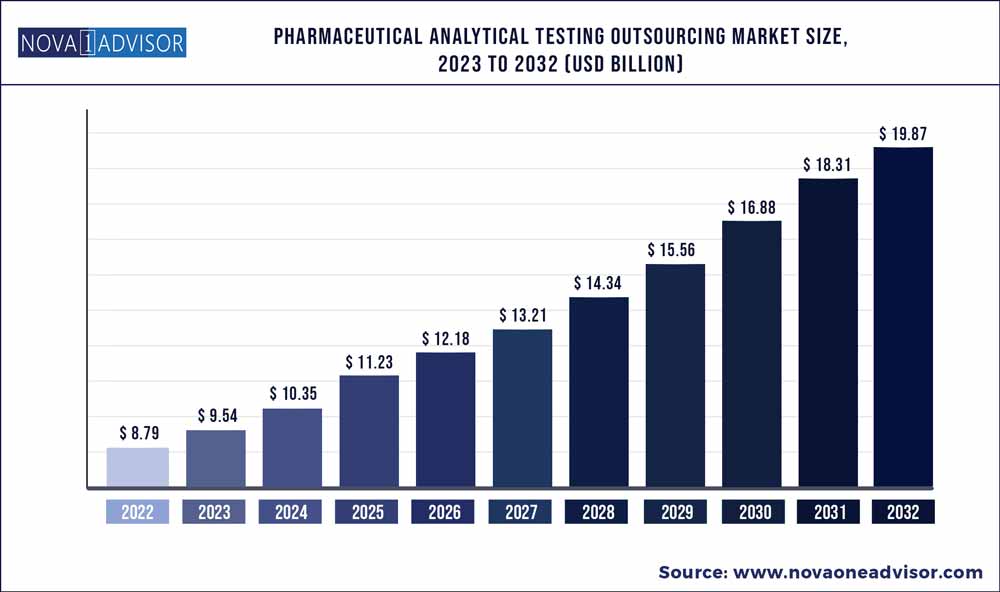
The global pharmaceutical analytical testing outsourcing market size was exhibited at USD 8.79 billion in 2022 and is projected to hit around USD 19.87 billion by 2032, growing at a CAGR of 8.5% during the forecast period 2023 to 2032.
Key Pointers:
- The other service segment accounted for a revenue share of over 40.9% in 2022.
- The pharmaceutical companies segment dominated the market for pharmaceutical analytical testing outsourcing and accounted for a revenue share of over 48.11% in 2022.
- The biopharmaceutical companies segment is expected to witness the fastest CAGR of 8.7% over the forecast.
- North America dominated the pharmaceutical analytical testing outsourcing market and accounted for the largest revenue share of over 54.9% in 2022
- Asia Pacific is anticipated to be the fastest-growing regional market from 2023 to 2032 due to the low-cost service offerings by the third-party service providers in the region
Pharmaceutical Analytical Testing Outsourcing Market Report Scope
|
Report Coverage
|
Details
|
|
Market Size in 2023
|
USD 9.54 Billion
|
|
Market Size by 2032
|
USD 19.87 Billion
|
|
Growth Rate from 2023 to 2032
|
CAGR of 8.5%
|
|
Base year
|
2022
|
|
Forecast period
|
2023 to 2032
|
|
Segments covered
|
Services, end-use
|
|
Regional scope
|
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa
|
|
Key companies profiled
|
SGS SA; Labcorp; Eurofins Scientific; Pace Analytical Services, Inc.; Intertek Group Plc; PPD Inc (Thermo Fisher Scientific, Inc); WuXi AppTec; Boston Analytical; Charles River Laboratories; West Pharmaceutical Services Inc.
|
The key factors driving the market are an innovation in the pharmaceutical industry, increasing focus on regulation, safety, and quality, the rising number of end-users, and the pricing benefits of outsourcing. Small and medium-sized pharmaceutical companies do not have the necessary infrastructure to support various types of analytical testing. As a result, outsourcing these processes is the best alternative as it saves time and money. The COVID-19 pandemic has increased the demand for pharmaceuticals. During this crisis, global pharmaceutical analytical testing outsourcing providers played a predominant role in meeting the testing needs of pharmaceutical companies, biotech companies, contract research organizations, and other end users.
These organizations have been actively working towards the development of pharmaceutical samples, APIs, small and large molecules, and other pharmaceutical products. The increase in the number of cases of COVID-19 is expected to improve the demand for these services, which is expected to have a positive impact on the market for pharmaceutical analytical testing outsourcing.
Other factors, such as the increasing need for product safety and quality, along with changing regulations for in vivo and in vitro tests, are also expected to propel the growth of the market for pharmaceutical analytical testing outsourcing. For instance, the government of China issued a notification to upgrade the quality of generic drugs, remove the backlog of drug applications, improve the quality and transparency of the review and approval process, and encourage new drug R&D in line with global development.
The economic benefits associated with outsourcing these services and approvals in the pharmaceutical industry are projected to support market growth. Quicker and more reliable results, data safety, and improved efficiency are some of the key factors favoring the market growth. Innovation or new product development is directly proportional to the demand for testing services. Due to competitive pressures, pricing concerns, and lead-time to market, companies are opting to outsource testing services. According to the Pharma Intelligence Report 2021, the total drugs in the pipeline increased to 18,852 molecules in 2021 from 17,737 molecules in 2020.
An increasing focus on customized care and technological advancements are shortening the product lifecycle, which has resulted in the rapid development of new products. The development of combination products, biosimilar, and other innovative medicines has led to an increase in demand for specific types of tests. Outsourcing services are becoming the most preferable option for many pharma companies to increase operational efficiency, therapeutic expertise, and on-demand services along with the expansion of geographical presence.
As per the WHO, the COVID-19 pandemic has significantly increased the demand for biological medicines like vaccines. As of April 15th, 2022, over 349 vaccines were under development as per the WHO. This is expected to improve the demand for analytical testing services for COVID-19 vaccines under clinical trials. Apart from this, several outsourcing companies have helped the pharma companies in testing drugs for COVID-19. For instance, Eurofins Scientific supported the testing of an investigational COVID-19 vaccine candidate by Johnson & Johnson. Similarly, SGS supported AstraZeneca and the University of Oxford to test the ingredients of their vaccine candidate. These actions by the outsourcing service providers are expected to have a positive impact on the market for pharmaceutical analytical testing outsourcing.
Some of the prominent players in the Pharmaceutical Analytical Testing Outsourcing Market include:
- SGS SA
- Labcorp
- Eurofins Scientific
- Pace Analytical Services, Inc.
- Intertek Group Plc
- PPD Inc. (Thermo Fisher Scientific)
- WuXi AppTec
- Boston Analytical
- Charles River Laboratories
- West Pharmaceutical Services Inc.
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Pharmaceutical Analytical Testing Outsourcing market.
By Services
- Bioanalytical
- Method Development & Validation
- Extractable & Leachable
- Impurity Method
- Technical Consulting
- Others
- Stability Testing
- Drug Substance
- Stability Indicating Method Validation
- Accelerated Stability Testing
- Photostability Testing
- Others
- Others
By End-use
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Contract Research Organizations
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
- Frequently Asked Questions

